In a comparison of Track and Trace versus Inspection and Authentication technologies it is apparent that Track and Trace solutions such as RFID, bar coding, holograms, tracing and pedigree products are not a one-size-fits-all solution to protecting the domestic and global pharmaceutical supply chain.
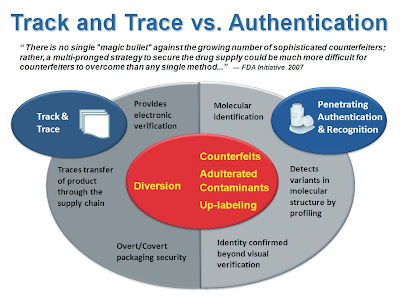
Track and Trace products might be sufficient, when employed throughout the Supply Chain to protect it from Drug Diversion which is one source of counterfeit activity, but these Track and Trace technologies cannot provide full protection for the product within the package. For example, how can anyone expect a Track and Trace product to determine if, either in the manufacturing or distribution process, the wrong ingredient was purposely or accidentally added into the product? Pedigree or Serialization may help, after a threat has propagated to the general public and was detected by other means, to help backtrack to the source of the problem. But by then it may be too late and the consumer will have already suffered.…
Only through the use of Inspection and Authentication technology such as XStream Systems’ XT250 Pharmaceutical Authentication System can those within the drug supply chain immediately inspect and authenticate the product within the container. Don’t let problems reach the general public. Stop the issues in their tracks.
For more information on XStream Systems and the XT250™ Pharmaceutical Authentication System, visit their website at www.xstreamsystems.net.









